<- previous index next ->
Many examples of spectra of molecules are presented on the
following pages. The spectra can be used to identify the
presents of specific molecules. Note that the same chemical
designation may result in more than one molecular structure
and the resulting spectra may be different.
Basic physics:
c = velocity of light = about 300,000,000 meters per second
f = frequency in Hz, Kilo KHz = 1000 Hz, Mega Mhz = 106Hz
Giga GHz = 109Hz Tera THz = 1012Hz
λ = wavelength in meters, μ = 10-6 meters = 1000 nano meters
c = λ * f
λ = c / f
f = c / λ
Example: wavelength 1 μ has frequency, f, computed by
f = 300*106 / 10-6 = 300*1012Hz = 300 THz
The "visible" spectrum, that which can be seen by average people,
is roughly given by wavelength in nm = nanometer or 10-9
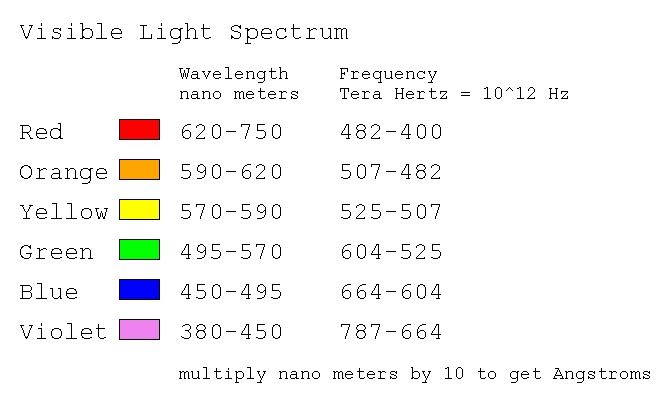 The following pages show the spectrum of many molecules.
The Infrared spectrum has wavelength μ in micrometers, 10-6 meters
The notation Frequency, cm-1 must be multiplied by 30*106
to get Hz.
The Nmr, Nuclear magnetic resonance, data is in Nmr units and Hz.
molecular.pdf Molecular frequency response
The following pages show the spectrum of many molecules.
The Infrared spectrum has wavelength μ in micrometers, 10-6 meters
The notation Frequency, cm-1 must be multiplied by 30*106
to get Hz.
The Nmr, Nuclear magnetic resonance, data is in Nmr units and Hz.
molecular.pdf Molecular frequency response
<- previous index next ->
-
CMSC 455 home page
-
Syllabus - class dates and subjects, homework dates, reading assignments
-
Homework assignments - the details
-
Projects -
-
Partial Lecture Notes, one per WEB page
-
Partial Lecture Notes, one big page for printing
-
Downloadable samples, source and executables
-
Some brief notes on Matlab
-
Some brief notes on Python
-
Some brief notes on Fortran 95
-
Some brief notes on Ada 95
-
An Ada math library (gnatmath95)
-
Finite difference approximations for derivatives
-
MATLAB examples, some ODE, some PDE
-
parallel threads examples
-
Reference pages on Taylor series, identities,
coordinate systems, differential operators
-
selected news related to numerical computation
 The following pages show the spectrum of many molecules.
The Infrared spectrum has wavelength μ in micrometers, 10-6 meters
The notation Frequency, cm-1 must be multiplied by 30*106
to get Hz.
The Nmr, Nuclear magnetic resonance, data is in Nmr units and Hz.
molecular.pdf Molecular frequency response
The following pages show the spectrum of many molecules.
The Infrared spectrum has wavelength μ in micrometers, 10-6 meters
The notation Frequency, cm-1 must be multiplied by 30*106
to get Hz.
The Nmr, Nuclear magnetic resonance, data is in Nmr units and Hz.
molecular.pdf Molecular frequency response